Ti-based Materials for Photoelectrochemical Water Splitting
Chin Wei Lai, Nur Azimah Abd Samad, Mohd Rafie Johan
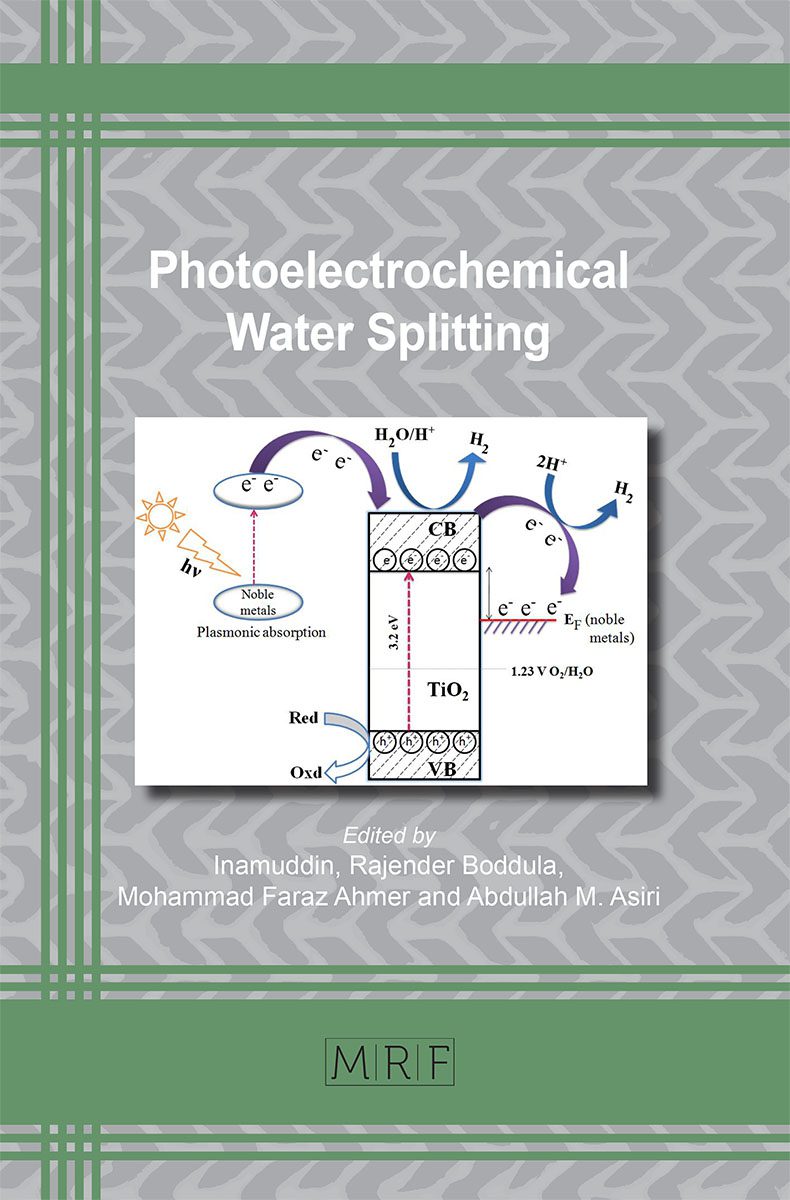
One of the most promising prospects for efficient renewable resources is the production of hydrogen (H2) gas. Extensive research on the development of n-type semiconductors for photoelectrochemical (PEC) water splitting process using solar energy is needed to bring H2 to the point of commercial readiness and viability in terms of performance and cost. Recent studies on TiO2 have recommended TiO2 as the leading candidate for PEC water splitting due to its low cost, non-toxicity, self-cleaning property, ready availability, strong photocatalytic activity, and stability against photocorrosion. In a PEC water splitting cell, the high efficiency of TiO2 as a photoelectrode requires an appropriate architecture that can minimize the loss of electrons at nanostructure connections and maximize photon absorption. To further improve the immigration of photo-induced charge carriers, considerable efforts are needed to further improve the performance of water splitting under visible light illumination. In the subsequent sections, the historical overview, basic principle, material selection and work done by various researchers with regards to the TiO2 based materials applied in PEC water splitting will be reviewed in detail.
Keywords
TiO2, Hydrogen, Photoelectrochemical Water Splitting, Photocatalytic Activity, Visible Light Illumination
Published online 3/5/2020, 19 pages
Citation: Chin Wei Lai, Nur Azimah Abd Samad, Mohd Rafie Johan, Ti-based Materials for Photoelectrochemical Water Splitting, Materials Research Foundations, Vol. 71, pp 110-128, 2020
DOI: https://doi.org/10.21741/9781644900734-5
Part of the book on Photoelectrochemical Water Splitting
References
[1] W. Kreuter, H. Hofmann, Electrolysis: the important energy transformer in a world of sustainable energy, Int. J. Hydrogen Energy 23 (1998) 661-666. https://doi.org/10.1016/S0360-3199(97)00109-2.
[2] V. Aroutiounian, V. Arakelyan, G. Shahnazaryan, Metal oxide photoelectrodes for hydrogen generation using solar radiation-driven water splitting, Sol. Energy 78 (2005) 581-592. https://doi.org/10.1016/j.solener.2004.02.002.
[3] D. Barreca, G. Carraro, V. Gombac, A. Gasparotto, C. Maccato, P. Fornasiero, E. Tondello, Supported metal oxide nanosystems for hydrogen photogeneration: Quo vadis?, Adv. Funct. Mater. 21 (2011) 2611-2623. https://doi.org/10.1002/adfm.201100242.
[4] H. Tong, S. Ouyang, Y. Bi, N. Umezawa, M. Oshikiri, J. Ye, Nano‐photocatalytic materials: possibilities and challenges, Adv. Mater. 24 (2012) 229-251. https://doi.org/10.1002/adma.201102752.
[5] D.M. Chapin, C. Fuller, G. Pearson, A new silicon p‐n junction photocell for converting solar radiation into electrical power, J. Appl. Phys. 25 (1954) 676-677. https://doi.org/10.1063/1.1721711.
[6] H. Okamoto, Y. Sugiyama, H. Nakano, Synthesis and modification of silicon nanosheets and other silicon nanomaterials, Chem. A Eur. J. 17 (2011) 9864-9887. https://doi.org/10.1002/chem.201100641.
[7] M. Grätzel, Photoelectrochemical cells, Nature 414 (2001) 338. https://doi.org/10.1038/35104607.
[8] A. Currao, Photoelectrochemical water splitting, CHIMIA Int. J. Chem. 61 (2007) 815-819. https://doi.org/10.2533/chimia.2007.815.
[9] C.A. Grimes, Synthesis and application of highly ordered arrays of TiO2 nanotubes, J. Mater. Chem. 17 (2007) 1451-1457. https://doi.org/10.1039/B701168G.
[10] N.K. Allam, C.A. Grimes, Effect of cathode material on the morphology and photoelectrochemical properties of vertically oriented TiO2 nanotube arrays, Sol. Energ. Mater. Sol. Cells 92 (2008) 1468-1475. https://doi.org/10.1016/j.solmat.2008.06.007.
[11] G. Centi, S. Perathoner, The role of nanostructure in improving the performance of electrodes for energy storage and conversion, Eur. J. Inorg. Chem. 2009 (2009) 3851-3878. https://doi.org/10.1002/ejic.200900275.
[12] A. Fujishima, K. Honda, Electrochemical photolysis of water at a semiconductor electrode, Nature 238 (1972) 37. https://doi.org/10.1038/238037a0.
[13] D.Y. Leung, X. Fu, C. Wang, M. Ni, M.K. Leung, X. Wang, X. Fu, Hydrogen production over titania‐based photocatalysts, ChemSusChem 3 (2010) 681-694. https://doi.org/10.1002/cssc.201000014.
[14] M. Ni, M.K. Leung, D.Y. Leung, K. Sumathy, A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production, Renewable and Sustainable Energy Reviews 11 (2007) 401-425. https://doi.org/10.1016/j.rser.2005.01.009.
[15] R.M. Navarro Yerga, M.C. Alvarez Galvan, F. Del Valle, J.A. Villoria de la Mano, J.L. Fierro, Water splitting on semiconductor catalysts under visible‐light irradiation, ChemSusChem 2 (2009) 471-485. https://doi.org/10.1002/cssc.200900018.
[16] M. Kitano, M. Matsuoka, M. Ueshima, M. Anpo, Recent developments in titanium oxide-based photocatalysts, Appl. Catal. A: Gen. 325 (2007) 1-14. https://doi.org/10.1016/j.apcata.2007.03.013.
[17] K. Shankar, G.K. Mor, M. Paulose, O.K. Varghese, C.A. Grimes, Effect of device geometry on the performance of TiO2 nanotube array-organic semiconductor double heterojunction solar cells, J. Non-Cryst. Solids 354 (2008) 2767-2771. https://doi.org/10.1016/j.jnoncrysol.2007.09.070.
[18] R. Dholam, N. Patel, M. Adami, A. Miotello, Physically and chemically synthesized TiO2 composite thin films for hydrogen production by photocatalytic water splitting, Int. J. Hydrogen Energy 33 (2008) 6896-6903. https://doi.org/10.1016/j.ijhydene.2008.08.061.
[19] J. Yan, F. Zhou, TiO 2 nanotubes: structure optimization for solar cells, J. Mater. Chem. 21 (2011) 9406-9418. https://doi.org/10.1039/C1JM10274E.
[20] L. Sun, S. Zhang, X. Sun, X. He, Effect of the geometry of the anodized titania nanotube array on the performance of dye-sensitized solar cells, J. Nanosci. Nanotechnol. 10 (2010) 4551-4561. https://doi.org/10.1166/jnn.2010.1695.
[21] P. Roy, S. Berger, P. Schmuki, TiO2 nanotubes: synthesis and applications, Angew. Chem. Int. Ed. 50 (2011) 2904-2939. https://doi.org/10.1002/anie.201001374.
[22] L. Yang, Y. Xiao, S. Liu, Y. Li, Q. Cai, S. Luo, G. Zeng, Photocatalytic reduction of Cr (VI) on WO3 doped long TiO2 nanotube arrays in the presence of citric acid, Appl. Catal. B: Environ. 94 (2010) 142-149. https://doi.org/10.1016/j.apcatb.2009.11.002.
[23] Z. Liu, Q. Zhang, T. Zhao, J. Zhai, L. Jiang, 3-D vertical arrays of TiO2 nanotubes on Ti meshes: efficient photoanodes for water photoelectrolysis, J. Mater. Chem. 21 (2011) 10354-10358. https://doi.org/10.1039/C1JM11072A.
[24] Y.C. Nah, I. Paramasivam, P. Schmuki, Doped TiO2 and TiO2 nanotubes: synthesis and applications, ChemPhysChem 11 (2010) 2698-2713. https://doi.org/10.1002/cphc.201000276.
[25] Z. Zhang, M.F. Hossain, T. Takahashi, Photoelectrochemical water splitting on highly smooth and ordered TiO2 nanotube arrays for hydrogen generation, Int. J. Hydrogen Energy 35 (2010) 8528-8535. https://doi.org/10.1016/j.ijhydene.2010.03.032.
[26] S. So, K. Lee, P. Schmuki, Ultrafast growth of highly ordered anodic TiO2 nanotubes in lactic acid electrolytes, J. Am. Chem. Soc. 134 (2012) 11316-11318. https://doi.org/10.1021/ja301892g.
[27] P. Hoyer, K. Nishio, H. Masuda, Preparation of regularly structured porous metal membranes with two different hole diameters at the two sides, Thin Solid Films 286 (1996) 88-91. https://doi.org/10.1016/S0040-6090(96)08549-5.
[28] X. Liu, J. Yang, L. Wang, X. Yang, L. Lu, X. Wang, An improvement on sol-gel method for preparing ultrafine and crystallized titania powder, Mater. Sci. Eng. A 289 (2000) 241-245. https://doi.org/10.1016/S0921-5093(00)00901-1.
[29] Z.-Y. Yuan, B.-L. Su, Titanium oxide nanotubes, nanofibers and nanowires, Colloids Surf. A 241 (2004) 173-183. https://doi.org/10.1016/j.colsurfa.2004.04.030.
[30] T. Kasuga, Formation of titanium oxide nanotubes using chemical treatments and their characteristic properties, Thin solid films 496 (2006) 141-145. https://doi.org/10.1016/j.tsf.2005.08.341.
[31] T. Kasuga, M. Hiramatsu, A. Hoson, T. Sekino, K. Niihara, Titania nanotubes prepared by chemical processing, Adv. Mater. 11 (1999) 1307-1311. https://doi.org/10.1002/(sici)1521-4095(199910)11:15<1307::aid-adma1307>3.0.co;2-h.
[32] M. Zhang, Z. Jin, J. Zhang, X. Guo, J. Yang, W. Li, X. Wang, Z. Zhang, Effect of annealing temperature on morphology, structure and photocatalytic behavior of nanotubed H2Ti2O4(OH)2, J. Molecular Catal. A: Chem 217 (2004) 203-210. https://doi.org/10.1016/j.molcata.2004.03.032.
[33] G. Mor, O.K. Varghese, M. Paulose, N. Mukherjee, C.A. Grimes, Fabrication of tapered, conical-shaped titania nanotubes, J. Mater. Res. 18 (2003) 2588-2593. https://doi.org/10.1557/JMR.2003.0362.
[34] P. Mishra, P. Shukla, A. Singh, O. Srivastava, Investigation and optimization of nanostructured TiO2 photoelectrode in regard to hydrogen production through photoelectrochemical process, Int. J. Hydrogen Energy 28 (2003) 1089-1094. https://doi.org/10.1016/S0360-3199(02)00197-0.
[35] G.K. Mor, O.K. Varghese, M. Paulose, K. Shankar, C.A. Grimes, A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications, Sol. Energ. Mater. and Sol. Cells 90 (2006) 2011-2075. https://doi.org/10.1016/j.solmat.2006.04.007.
[36] M. Paulose, G.K. Mor, O.K. Varghese, K. Shankar, C.A. Grimes, Visible light photoelectrochemical and water-photoelectrolysis properties of titania nanotube arrays, J. Photochem. Photobio. A: Chem.178 (2006) 8-15. https://doi.org/10.1016/j.jphotochem.2005.06.013.
[37] J.M. Macak, P. Schmuki, Anodic growth of self-organized anodic TiO2 nanotubes in viscous electrolytes, Electrochim. Acta 52 (2006) 1258-1264. https://doi.org/10.1016/j.electacta.2006.07.021.
[38] A. Ghicov, P. Schmuki, Self-ordering electrochemistry: a review on growth and functionality of TiO 2 nanotubes and other self-aligned MOx structures, Chem. Commun. (2009) 2791-2808. https://doi.org/10.1039/B822726H.
[39] S. Komornicki, M. Radecka, P. Sobaś, Structural, electrical and optical properties of TiO2–WO3 polycrystalline ceramics, Mater. Res. Bull. 39 (2004) 2007-2017. https://doi.org/10.1016/j.materresbull.2004.07.017.
[40] S. Higashimoto, M. Sakiyama, M. Azuma, Photoelectrochemical properties of hybrid WO3/TiO2 electrode. Effect of structures of WO3 on charge separation behavior, Thin Solid Films 503 (2006) 201-206. https://doi.org/10.1016/j.tsf.2005.11.110.
[41] J.H. Bang, P.V. Kamat, Solar cells by design: photoelectrochemistry of TiO2 nanorod arrays decorated with CdSe, Adv. Funct. Mater. 20 (2010) 1970-1976. https://doi.org/10.1002/adfm.200902234.
[42] J. Zhang, Y. Wu, M. Xing, S.A.K. Leghari, S. Sajjad, Development of modified N doped TiO 2 photocatalyst with metals, nonmetals and metal oxides, Energy Environ. Sci. 3 (2010) 715-726. https://doi.org/10.1039/B927575D.
[43] H.-i. Kim, J. Kim, W. Kim, W. Choi, Enhanced photocatalytic and photoelectrochemical activity in the ternary hybrid of CdS/TiO2/WO3 through the cascadal electron transfer, J. Phys. Chem. C 115 (2011) 9797-9805. https://doi.org/10.1021/jp1122823.
[44] K. Maeda, A. Xiong, T. Yoshinaga, T. Ikeda, N. Sakamoto, T. Hisatomi, M. Takashima, D. Lu, M. Kanehara, T. Setoyama, Photocatalytic overall water splitting promoted by two different cocatalysts for hydrogen and oxygen evolution under visible light, Angew. Chem. Int. Ed. 49 (2010) 4096-4099. https://doi.org/10.1002/anie.201001259.
[45] G. Korotcenkov, S. Han, B. Cho, Metal oxide nanocomposites: Advantages and shortcomings for application in conductometric gas sensors, Materials Science Forum, Trans. Tech. Publ. (2016) 223-229. https://doi.org/10.4028/www.scientific.net/MSF.872.223.
[46] M. Takeuchi, H. Yamashita, M. Matsuoka, M. Anpo, T. Hirao, N. Itoh, N. Iwamoto, Photocatalytic decomposition of NO under visible light irradiation on the Cr‐ion‐implanted TiO2 thin film photocatalyst, Catal. Lett. 67 (2000) 135-137. https://doi.org/10.1023/A:1019065521567.
[47] E. Piera, M.I. Tejedor-Tejedor, M.E. Zorn, M.A. Anderson, Relationship concerning the nature and concentration of Fe (III) species on the surface of TiO2 particles and photocatalytic activity of the catalyst, Appl. Catal. B: Environ. 46 (2003) 671-685. https://doi.org/10.1016/S0926-3373(03)00288-1.
[48] K. Miyashita, S.-i. Kuroda, S. Tajima, K. Takehira, S. Tobita, H. Kubota, Photoluminescence study of electron–hole recombination dynamics in the vacuum-deposited SiO2/TiO2 multilayer film with photo-catalytic activity, Chem. Phys. Lett. 369 (2003) 225-231. https://doi.org/10.1016/S0009-2614(02)02009-2.
[49] X. Zhang, K. Huo, L. Hu, P.K. Chu, Fabrication and photocatalytic activity of nanoporous WO3 film, Nanosci. Nanotechnol. Lett. 2 (2010) 51-57. https://doi.org/10.1166/nnl.2010.1052.
[50] J.C.-S. Wu, C.-H. Chen, A visible-light response vanadium-doped titania nanocatalyst by sol–gel method, J. Photochem. Photobio. A: Chem. 163 (2004) 509-515. https://doi.org/10.1016/j.jphotochem.2004.02.007.
[51] R. Sonawane, M. Dongare, Sol–gel synthesis of Au/TiO2 thin films for photocatalytic degradation of phenol in sunlight, J. Molecular Catal. A: Chem. 243 (2006) 68-76. https://doi.org/10.1016/j.molcata.2005.07.043.
[52] A. Mirescu, H. Berndt, A. Martin, U. Prüße, Long-term stability of a 0.45% Au/TiO2 catalyst in the selective oxidation of glucose at optimised reaction conditions, Appl. Catal. A: Gen. 317 (2007) 204-209. https://doi.org/10.1016/j.apcata.2006.10.016.
[53] A.V. Rupa, D. Manikandan, D. Divakar, T. Sivakumar, Effect of deposition of Ag on TiO2 nanoparticles on the photodegradation of Reactive Yellow-17, J. Hazardous Mater. 147 (2007) 906-913. https://doi.org/10.1016/j.jhazmat.2007.01.107.
[54] Y.J. Choi, Z. Seeley, A. Bandyopadhyay, S. Bose, S.A. Akbar, Aluminum-doped TiO2 nano-powders for gas sensors, Sensors and Actuators B: Chem. 124 (2007) 111-117. https://doi.org/10.1016/j.snb.2006.12.005.
[55] H. Li, X. Duan, G. Liu, L. Li, Synthesis and characterization of copper ions surface-doped titanium dioxide nanotubes, Mater. Res. Bull. 43 (2008) 1971-1981. https://doi.org/10.1016/j.materresbull.2007.10.005.
[56] N. Sobana, K. Selvam, M. Swaminathan, Optimization of photocatalytic degradation conditions of Direct Red 23 using nano-Ag doped TiO2, Sep. Purif. Technol. 62 (2008) 648-653. https://doi.org/10.1016/j.seppur.2008.03.002.
[57] S.K. Mohapatra, N. Kondamudi, S. Banerjee, M. Misra, Functionalization of self-organized TiO2 nanotubes with Pd nanoparticles for photocatalytic decomposition of dyes under solar light illumination, Langmuir 24 (2008) 11276-11281. https://doi.org/10.1021/la801253f.
[58] S. Kuang, L. Yang, S. Luo, Q. Cai, Fabrication, characterization and photoelectrochemical properties of Fe2O3 modified TiO2 nanotube arrays, Appl. Surf. Sci. 255 (2009) 7385-7388. https://doi.org/10.1016/j.apsusc.2009.04.005.
[59] Y. Hou, X.Y. Li, Q. Zhao, X. Quan, G. Chen, Fabrication of Cu2O/TiO2 nanotube heterojunction arrays and investigation of its photoelectrochemical behavior, Appl. Phys. Lett. 95 (2009) 093108. https://doi.org/10.1063/1.3224181.
[60] Y. Hou, X.Y. Li, Q.D. Zhao, X. Quan, G.H. Chen, Electrochemical method for synthesis of a ZnFe2O4/TiO2 composite nanotube array modified electrode with enhanced photoelectrochemical activity, Adv. Funct. Mater. 20 (2010) 2165-2174. https://doi.org/10.1002/adfm.200902390.
[61] Y. Liu, H. Zhou, J. Li, H. Chen, D. Li, B. Zhou, W. Cai, Enhanced photoelectrochemical properties of Cu2O-loaded short TiO2 nanotube array electrode prepared by sonoelectrochemical deposition, Nano-Micro Lett. 2 (2010) 277-284. https://doi.org/10.3786/nml.v2i4.p277-284.
[62] D. Lofficial, A. Fecant, D. Uzio, E. Puzenat, C. Geantet, Synthesis and characterisation of Cu2O/Pt/TiO2 hybrid materials for photocatalytic valorization of CO2, Photocatalysis for energy [PHOTO4E], 2014.
[63] C. Chen, W. Cai, M. Long, B. Zhou, Y. Wu, D. Wu, Y. Feng, Synthesis of visible-light responsive graphene oxide/TiO2 composites with p/n heterojunction, ACS Nano 4 (2010) 6425-6432. https://doi.org/10.1021/nn102130m
[64] H. Tada, Q. Jin, H. Nishijima, H. Yamamoto, M. Fujishima, S.i. Okuoka, T. Hattori, Y. Sumida, H. Kobayashi, Titanium (IV) dioxide surface‐modified with iron oxide as a visible light photocatalyst, Angew. Chem. Int. Ed. 50 (2011) 3501-3505. https://doi.org/10.1002/anie.201007869.
[65] W. Wu, X. Xiao, S. Zhang, F. Ren, C. Jiang, Facile method to synthesize magnetic iron oxides/TiO2 hybrid nanoparticles and their photodegradation application of methylene blue, Nanoscale Res. Lett. 6 (2011) 533. https://doi.org/10.1186/1556-276X-6-533.
[66] S. Chaguetmi, N. Sobti, P. Decorse, L. Mouton, S. Nowak, F. Mammeri, S. Achour, S. Ammar, Visible-light photocatalytic performances of TiO2 nanobelts decorated with iron oxide nanocrystals, RSC Adv. 6 (2016) 114843-114851. https://doi.org/10.1039/C6RA24415G.
[67] J. Liu, J. Ke, Y. Li, B. Liu, L. Wang, H. Xiao, S. Wang, Co3O4 quantum dots/TiO2 nanobelt hybrids for highly efficient photocatalytic overall water splitting, Appl. Catal. B: Environ. 236 (2018) 396-403. https://doi.org/10.1016/j.apcatb.2018.05.042.
[68] K. Arifin, H.A. Kadir, L.J. Minggu, W.R.W. Daud, M.B. Kassim, TiO2 doped with Fe2O3 for photoelectrochemical water splitting electrode: experimental and density functional theory study, Malaysian J. Anal. Sci. 20 (2016) 892-900. https://doi.org/10.17576/mjas-2016-2004-25.
[69] M.A. Mansoor, M. Mazhar, V. McKee, Z. Arifin, Mn2O3–TiO2 semiconducting composite thin films for photo-electrochemical water splitting, Polyhedron 75 (2014) 135-140. https://doi.org/10.1016/j.poly.2014.03.018.