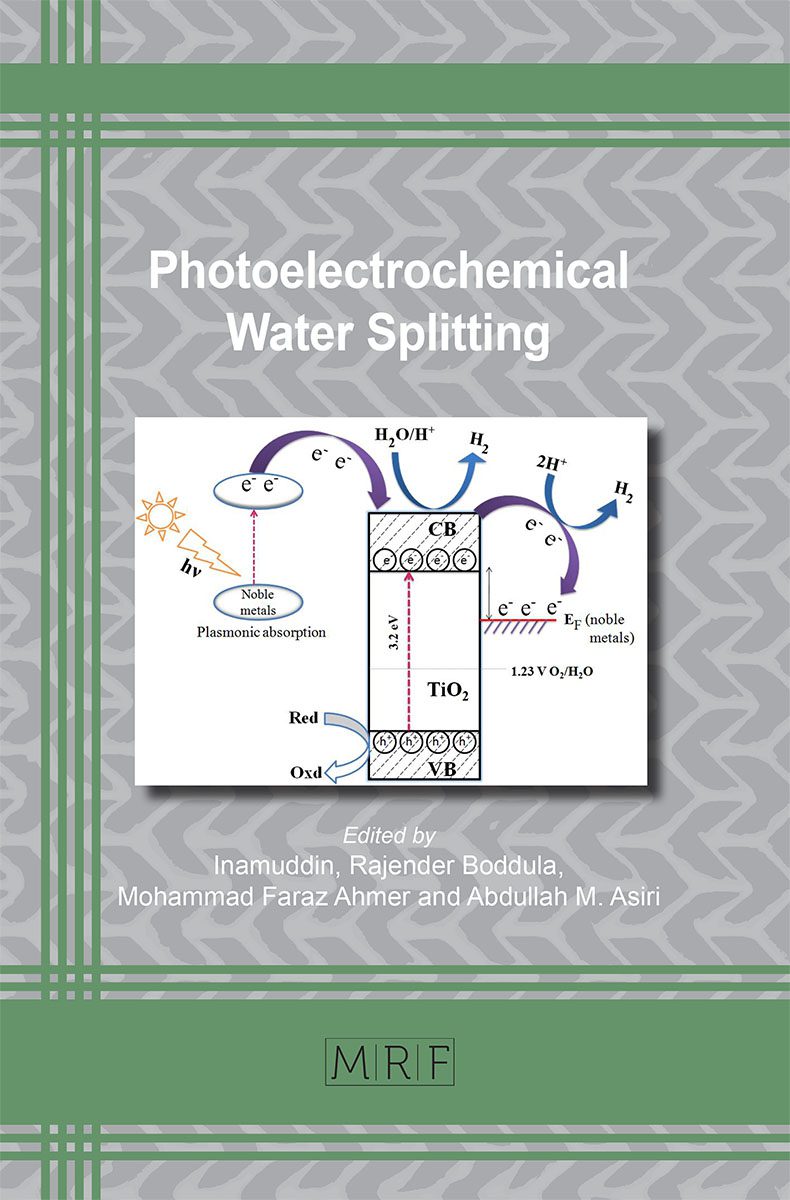
Narrow Bandgap Semiconductors for Photoelectrochemical Water Splitting
Sonal Singh, Rishabh Sharma and Manika Khanuja
With the aim of directing research towards the area of water splitting using photoelectrochemical (PEC) cell, it is necessary to optimize the semiconductor materials used as photoelectrodes in PEC system. Narrow band gap materials prove to ace the race due to their several favorable properties towards solar absorption and water splitting. Also, their energy and potential dynamics seem suitable for this particular application. Although, many narrow band gap materials are available to cause complete splitting of water, certain shortcomings limit their full potential efficiency. To overcome these, certain alterations or modifications are required through some strategies in the form of doping, composites, heterojunctions, and coupling etc. Coupling of narrow band gap materials with wide bandgap materials has proved extremely beneficial for both the counterparts in overcoming the drawbacks of both the materials in a composite and ultimately improving the overall efficiency of the PEC device.
Keywords
Narrow Band Gap Materials, Photoelectrochemical, Water Splitting, Coupling
Published online 3/5/2020, 19 pages
Citation: Sonal Singh, Rishabh Sharma and Manika Khanuja, Narrow Bandgap Semiconductors for Photoelectrochemical Water Splitting, Materials Research Foundations, Vol. 71, pp 91-109, 2020
DOI: https://doi.org/10.21741/9781644900734-4
Part of the book on Photoelectrochemical Water Splitting
References
[1] S. Singh, S. Jain, V. PS, A.K. Tiwari, M.R. Nouni, J.K. Pandey, S. Goel, Hydrogen: A sustainable fuel for future of the transport sector, Renew. Sustain. Energy Rev. 51 (2015) 623–633. https://doi.org/10.1016/j.rser.2015.06.040
[2] N.S.A. Case, B.S. Bi, Z. Zhang, W. Wang, L. Wang, S. Sun, Enhancement of visible-light photocatalysis by coupling with narrow-band-gap semiconductor: A Case Study on Bi2S3/Bi2WO6, (2012) 593–597. https://doi.org/10.1021/am2017199
[3] R. Suarez, P.K. Nair, P. V Kamat, Photoelectrochemical behavior of Bi2S3 nanoclusters and nanostructured thin films, Langmuir 12 (1998) 3236–3241. https://doi.org/10.1021/la9801662
[4] A.A. Tahir, M.A. Ehsan, M. Mazhar, K.G.U. Wijayantha, M. Zeller, A.D. Hunter, Photoelectrochemical and photoresponsive properties of Bi2S3 nanotube and nanoparticle thin films, (2010) 5084–5092. https://doi.org/10.1021/cm101642b
[5] Y. Bessekhouad, D. Robert, J. V Weber, Bi2S3/TiO2 and CdS/TiO2 heterojunctions as an available configuration for photocatalytic degradation of organic pollutant, J. Photochem. Photobiol. A Chem. 163 (2004) 569–580. https://doi.org/10.1016/j.jphotochem.2004.02.006
[6] A. Jana, C. Bhattacharya, J. Datta, Enhanced photoelectrochemical activity of electro-synthesized CdS–Bi2S3 composite films grown with self-designed cross-linked structure, Electrochim. Acta 55 (2010) 6553–6562. https://doi.org/10.1016/j.electacta.2010.06.022
[7] X. Lu, F. Pu, Y. Xia, W. Huang, Z. Li, Facile fabrication of porous thin films of Bi2O3/Bi2S3 nanocomposite semiconductors at gas/liquid interface and their photoelectrochemical performances, Appl. Surf. Sci. 299 (2014) 131–135. https://doi.org/10.1016/j.apsusc.2014.01.196
[8] X. Gao, H. Bin Wu, L. Zheng, Y. Zhong, Y. Hu, X.W. Lou, Formation of mesoporous heterostructured BiVO4/Bi2S3 hollow discoids with enhanced photoactivity, Angew. Chemie Int. Ed. 126 (2014) 6027–6031. https://doi.org/10.1002/ange.201403611
[9] C. Liu, Y. Yang, W. Li, J. Li, Y. Li, Q. Chen, Construction of novel Bi2S3 nanobelt@WO3 nanoplate arrays on FTO glass with high photoelectrochemical activity, Int. J. Hydrogen Energy 41 (2016) 5878–5886. https://doi.org/10.1016/j.ijhydene.2016.01.171
[10] G. Ai, R. Mo, Q. Chen, H. Xu, S. Yang, H. Li, J. Zhong, TiO2/Bi2S3 core–shell nanowire arrays for photoelectrochemical hydrogen generation, RSC Adv. 5 (2015) 13544–13549. https://doi.org/10.1039/C4RA15820B
[11] Y. Wan, M. Han, L. Yu, J. Jia, G. Yi, Fabrication and photoelectrochemical properties of TiO2/CuInS2/Bi2S3 core/shell/shell nanorods electrodes, RSC Adv. 5 (2015) 78902–78909. https://doi.org/10.1039/C5RA14548A
[12] F.-A. Liu, Y.-C. Yang, J. Liu, W. Huang, Z.-L. Li, Preparation of Bi2O3@ Bi2S3 core–shell nanoparticle assembled thin films and their photoelectrochemical and photoresponsive properties, J. Electroanal. Chem. 665 (2012) 58–62. https://doi.org/10.1016/j.jelechem.2011.11.015
[13] X. Rong, F. Qiu, J. Yan, H. Zhao, X. Zhu, D. Yang, Coupling with a narrow-band-gap semiconductor for enhancement of visible-light photocatalytic activity: preparation of Bi2S3/g-C3N4 and application for degradation of RhB, RSC Adv. 5 (2015) 24944-24952. https://doi.org/10.1039/C4RA15715J
[14] O.K. Okoth, K. Yan, Y. Liu, J. Zhang, Graphene-doped Bi2S3 nanorods as visible-light photoelectrochemical aptasensing platform for sulfadimethoxine detection, Biosens. Bioelectron. 86 (2016) 636–642. https://doi.org/10.1016/j.bios.2016.07.037
[15] D. Chauhan, V.R. Satsangi, S. Dass, R. Shrivastav, Preparation and characterization of nanostructured CuO thin films for photoelectrochemical splitting of water, Bull. Mater. Sci. 29 (2006) 709–716.
[16] G. Wang, H. Wang, Y. Ling, Y. Tang, X. Yang, R.C. Fitzmorris, C. Wang, J.Z. Zhang, Y. Li, Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting, Nano Lett. 11 (2011) 3026–3033. https://doi.org/10.1021/nl201766h
[17] Y. Ling, G. Wang, J. Reddy, C. Wang, J.Z. Zhang, Y. Li, The influence of oxygen content on the thermal activation of hematite nanowires, Angew. Chemie Int. Ed. 124 (2012) 4150–4155. https://doi.org/10.1002/ange.201107467
[18] M.P. Dare-Edwards, J.B. Goodenough, A. Hamnett, P.R. Trevellick, Electrochemistry and photoelectrochemistry of iron (III) oxide, J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases. 79 (1983) 2027–2041. https://doi.org/10.1039/f19837902027
[19] P. Salvador, Hole diffusion length in n‐TiO2 single crystals and sintered electrodes: Photoelectrochemical determination and comparative analysis, J. Appl. Phys. 55 (1984) 2977–2985. https://doi.org/10.1063/1.333358
[20] E. Hendry, M. Koeberg, B. O’regan, M. Bonn, Local field effects on electron transport in nanostructured TiO2 revealed by terahertz spectroscopy, Nano Lett. 6 (2006) 755–759. https://doi.org/10.1021/nl0600225
[21] A. Kargar, Y. Jing, S.J. Kim, C.T. Riley, X. Pan, D. Wang, ZnO/CuO Heterojunction branched nanowires for photoelectrochemical hydrogen generation, ACS Nano (2013) 11112–11120. https://doi.org/10.1021/nn404838n
[22] I.S. Cho, Z. Chen, A.J. Forman, D.R. Kim, P.M. Rao, T.F. Jaramillo, X. Zheng, Branched TiO2 nanorods for photoelectrochemical hydrogen production, Nano Lett. 11 (2011) 4978–4984. https://doi.org/10.1021/nl2029392
[23] X. Yang, A. Wolcott, G. Wang, A. Sobo, R.C. Fitzmorris, F. Qian, J.Z. Zhang, Y. Li, Nitrogen-doped ZnO nanowire arrays for photoelectrochemical water splitting, Nano Lett. 9 (2009) 2331–2336. https://doi.org/10.1021/nl900772q
[24] W. Siripala, A. Ivanovskaya, T.F. Jaramillo, S.-H. Baeck, E.W. McFarland, A Cu2O/TiO2 heterojunction thin film cathode for photoelectrocatalysis, Sol. Energy Mater. Sol. Cells. 77 (2003) 229–237. https://doi.org/10.1016/S0927-0248(02)00343-4
[25] X. Guo, P. Diao, D. Xu, S. Huang, Y. Yang, T. Jin, Q. Wu, M. Xiang, M. Zhang, ScienceDirect CuO/Pd composite photocathodes for photoelectrochemical hydrogen evolution reaction, (2014) 1–11. https://doi.org/10.1016/j.ijhydene.2014.03.084
[26] F.P. Koffyberg, F.A. Benko, A photoelectrochemical determination of the position of the conduction and valence band edges of p‐type CuO, J. Appl. Phys. 53 (1982) 1173–1177. https://doi.org/10.1063/1.330567
[27] H.-J. Choi, M. Kang, Hydrogen production from methanol/water decomposition in a liquid photosystem using the anatase structure of Cu loaded TiO2, Int. J. Hydrogen Energy 32 (2007) 3841–3848. https://doi.org/10.1016/j.ijhydene.2007.05.011
[28] C. Chiang, Y. Shin, S. Ehrman, Li Doped CuO Film Electrodes for Photoelectrochemical Cells, J. Electrochem. Soc. 159 (2012) 227–231. https://doi.org/10.1149/2.081202jes
[29] S. Choudhary, A. Solanki, S. Upadhyay, N. Singh, V.R. Satsangi, R. Shrivastav, S. Dass, Nanostructured CuO/SrTiO3 bilayered thin films for photoelectrochemical water splitting, J. Solid State Electrochem. 17 (2013) 2531–2538. https://doi.org/10.1007/s10008-013-2139-7
[30] H.S. Park, C.-Y. Lee, E. Reisner, Photoelectrochemical reduction of aqueous protons with a CuO|CuBi2O4 heterojunction under visible light irradiation, Phys. Chem. Chem. Phys. 16 (2014) 22462–22465. https://doi.org/10.1039/C4CP03883E
[31] J.Y. Zheng, G. Song, C.W. Kim, Y.S. Kang, Facile preparation of p-CuO and p-CuO/n-CuWO4 junction thin films and their photoelectrochemical properties, Electrochim. Acta 69 (2012) 340–344. https://doi.org/10.1016/j.electacta.2012.03.011
[32] A.A. Dubale, C.J. Pan, A.G.Tamirat, H.M. Chen, W.N.Su, C.H. Chen, J. Rick, D.W. Ayele, B.A. Aragaw, J.F. Lee, Y.W.Yang, B.J. Hwang, Heterostructured Cu2O/CuO decorated with nickel as a highly efficient photocathode for photoelectrochemical water reduction, J. Mater. Chem. A 3 (2015) 12482-12499. https://doi.org/10.1039/C5TA01961C
[33] A.A. Tahir, K.G. Upul Wijayantha, S. Saremi-Yarahmadi, M. Maznar, V. McKee, Nanostructured α-Fe2O3 thin films for photoelectrochemical hydrogen generation, Chem. Mater. 21 (2009) 3763–3772. https://doi.org/10.1021/cm803510v
[34] V.M. Aroutiounian, V.M. Arakelyan, G.E. Shahnazaryan, G.M. Stepanyan, E.A. Khachaturyan, H. Wang, J.A. Turner, Photoelectrochemistry of semiconductorelectrodes made of solid solutions in the system Fe2O3–Nb2O5, Sol. Energy. 80 (2006) 1098–1111. https://doi.org/10.1016/j.solener.2005.10.005
[35] A.B. Murphy, P.R.F. Barnes, L.K. Randeniya, I.C. Plumb, I.E. Grey, M.D. Horne, J.A. Glasscock, Efficiency of solar water splitting using semiconductor electrodes, Int. J. Hydrogen Energy. 31 (2006) 1999–2017. https://doi.org/10.1016/j.ijhydene.2006.01.014
[36] T. Arai, Y. Konishi, Y. Iwasaki, H. Sugihara, K. Sayama, High-throughput screening using porous photoelectrode for the development of visible-light-responsive semiconductors, J. Comb. Chem. 9 (2007) 574–581. https://doi.org/10.1021/cc0700142
[37] R. Shinar, J.H. Kennedy, Photoactivity of doped α-Fe2O3 electrodes, Sol. Energy Mater. 6 (1982) 323–335. https://doi.org/10.1016/0165-1633(82)90038-7
[38] C. Leygraf, M. Hendewerk, G.A. Somorjai, Photodissociation of water by p-and n-type polycrystalline iron oxides by using visible light (≤ 2.7 eV) in the absence of external potential, Proc. Natl. Acad. Sci. 79 (1982) 5739–5741. https://doi.org/10.1073/pnas.79.18.5739
[39] U. Bjoerksten, J. Moser, M. Graetzel, Photoelectrochemical studies on nanocrystalline hematite films, Chem. Mater. 6 (1994) 858–863. https://doi.org/10.1021/cm00042a026
[40] C. Jorand Sartoretti, B.D. Alexander, R. Solarska, I.A. Rutkowska, J. Augustynski, R. Cerny, Photoelectrochemical oxidation of water at transparent ferric oxide film electrodes, J. Phys. Chem. B. 109 (2005) 13685–13692. https://doi.org/10.1021/jp051546g
[41] W.D. Chemelewski, N.T. Hahn, C.B. Mullins, Effect of Si doping and porosity on hematite’s (α-Fe2O3) photoelectrochemical water oxidation performance, J. Phys. Chem. C. 116 (2012) 5255–5261. https://doi.org/10.1021/jp210877u
[42] Y.-S. Hu, A. Kleiman-Shwarsctein, A.J. Forman, D. Hazen, J.-N. Park, E.W. McFarland, Pt-doped α-Fe2O3 thin films active for photoelectrochemical water splitting, Chem. Mater. 20 (2008) 3803–3805. https://doi.org/10.1021/cm800144q
[43] N.T. Hahn, C.B. Mullins, Photoelectrochemical performance of nanostructured Ti- and Sn-doped α-Fe2O3 photoanodes, Chem. Mater. 22 (2010) 6474–6482. https://doi.org/10.1021/cm1026078
[44] Y. Hou, F. Zuo, A. Dagg, P. Feng, Visible Light-Driven α-Fe2O3 Nanorod/Graphene/BiV1–xMoxO4 Core/Shell Heterojunction Array for Efficient Photoelectrochemical Water Splitting, Nano Lett. 12 (2012) 6464-6473. https://doi.org/10.1021/nl303961c
[45] Y. Lei, G. Wang, S. Song, W. Fan, M. Pang, J. Tang, H. Zhang, Room temperature, template-free synthesis of BiOI hierarchical structures: visible-light photocatalytic and electrochemical hydrogen storage properties, Dalton Trans. 39 (2010) 3273–3278. https://doi.org/10.1039/b922126c
[46] S. Singh, R. Sharma, M. Khanuja, A review and recent developments on strategies to improve the photocatalytic elimination of organic dye pollutants by BiOX ( X = Cl , Br, I , F ) nanostructures, Korean J. Chem. Eng. 35 (2018) 1955-1968. https://doi.org/10.1007/s11814-018-0112-y
[47] H. Lin, C. Zhou, J. Cao, S. Chen, Ethylene glycol-assisted synthesis, photoelectrochemical and photocatalytic properties of BiOI microflowers, Chinese Sci. Bull. 59 (2014) 3420–3426. https://doi.org/10.1007/s11434-014-0433-0
[48] N.T. Hahn, S. Hoang, J.L. Self, C.B. Mullins, Spray pyrolysis deposition and photoelectrochemical properties of n-Type BiOI nanoplatelet thin films, ACS Nano 6 (2012) 7712–7722. doi:10.1021/nn3031063. https://doi.org/10.1021/nn3031063
[49] L. Shan, L. He, J. Suriyaprakash, L. Yang, Photoelectrochemical (PEC) water splitting of BiOI {001} nanosheets synthesized by a simple chemical transformation, J. Alloys Compd. 665 (2016) 158–164. https://doi.org/10.1016/j.jallcom.2016.01.008
[50] S. Xie, K. Ouyang, X. Ma, Low temperature synthesis of plate-like BiOIs and their highly enhanced visible light photocatalytic performance, Ceram. Int. 40 (2014) 12353–12357. https://doi.org/10.1016/j.ceramint.2014.04.081
[51] Q.C. Liu, D.K. Ma, Y.Y. Hu, Y.W. Zeng, S.M. Huang, Various bismuth oxyiodide hierarchical architectures: Alcohothermal- controlled synthesis, photocatalytic activities, and adsorption capabilities for phosphate in water, ACS Appl. Mater. Interfaces 5 (2013) 11927–11934. https://doi.org/10.1021/am4036702
[52] Y. Mi, M. Zhou, L. Wen, H. Zhao, Y. Lei, 6-A highly efficient visible-light driven photocatalyst: two dimensional square-like bismuth oxyiodine nanosheets, Dalton Trans. 43 (2014) 9549–9556. https://doi.org/10.1039/C4DT00798K
[53] X. Xiao, W.-D. Zhang, Facile synthesis of nanostructured BiOI microspheres with high visible light-induced photocatalytic activity, J. Mater. Chem. 20 (2010) 5866–5870. https://doi.org/10.1039/c0jm00333f
[54] M. Fang, H. Jia, W. He, Y. Lei, L. Zhang, Z. Zheng, Construction of flexible photoelectrochemical solar cells based on ordered nanostructural BiOI/Bi2S3 heterojunction films, Phys. Chem. Chem. Phys. 17 (2015) 13531–13538. https://doi.org/10.1039/C4CP05749J
[55] A. Malathi, P. Arunachalam, A.N. Grace, J. Madhavan, A.M. Al-Mayouf, A robust visible-light driven BiFeWO6/BiOI nanohybrid with efficient photocatalytic and photoelectrochemical performance, Appl. Surf. Sci. 412 (2017) 85–95. https://doi.org/10.1016/j.apsusc.2017.03.199
[56] H. Lin, H. Ye, X. Li, J. Cao, S. Chen, Facile anion-exchange synthesis of BiOI/BiOBr composite with enhanced photoelectrochemical and photocatalytic properties, Ceram. Int. 40 (2014) 9743–9750. https://doi.org/10.1016/j.ceramint.2014.02.060
[57] R.S. Silva, H.D. Mikhail, N.F. Cano, N.O. Dantas, Nanocrystals embedded in a glass matrix, Molecules 22 (2017) 1142. https://doi.org/10.3390/molecules22071142
[58] B. Wang, Z. Song, B. Wang, J. Yu, C. Ma, C. Zhou, T. Chen, Density functional study on the heterogeneous oxidation of NO over α-Fe2O3 catalyst by H2O2: Effect of oxygen vacancy, Appl. Surf. Sci. 413 (2017) 292–301. https://doi.org/10.1016/j.apsusc.2017.04.011
[59] W. Dai, Z. Zhao, Electronic Structure and Optical Properties of BiOI as a Photocatalyst Driven by Visible Light, Inorg. Chem. 54 (2016) 10732–10737. https://doi.org/10.1021/acs.inorgchem.5b01714
[60] R.S. Mane, B.R. Sankapal, C.D. Lokhande, Photoelectrochemical cells based on chemically deposited nanocrystalline Bi2S3 thin films, Mater. Chem. Phys. 60 (1999) 196–203. https://doi.org/10.1016/S0254-0584(99)00085-1
[61] R. Brahimi, Y. Bessekhouad, A. Bouguelia, M. Trari, Visible light induced hydrogen evolution over the heterosystem Bi2S3/TiO2, Catal. Today. 122 (2007) 62–65. https://doi.org/10.1016/j.cattod.2007.01.030
[62] Q. Zeng, J. Bai, J. Li, Y. Li, X. Li, B. Zhou, Combined nanostructured Bi2S3/TNA photoanode and Pt/SiPVC photocathode for efficient self-biasing photoelectrochemical hydrogen and electricity generation, Nano Energy. 9 (2014) 152–160. https://doi.org/10.1016/j.nanoen.2014.06.023
[63] K.M. Gadave, C.D. Lokhande, P.P. Hankare, Characterization of CdS-Bi2S3 pellets prepared by co-precipitation method, Mater. Chem. Phys. 38 (1994) 393–397. https://doi.org/10.1016/0254-0584(94)90219-4
[64] D.K. Zhong, D.R. Gamelin, Photoelectrochemical water oxidation by cobalt catalyst (“ Co – Pi ”)/α-Fe2O3 Composite Photoanodes : Oxygen Evolution and resolution of a kinetic bottleneck, J. Am. Chem. Soc.132 (2010) 4202–4207. https://doi.org/10.1021/ja908730h
[65] S.U.M. Khan, J. Akikusa, Photoelectrochemical splitting of water at nanocrystalline n-Fe2O3 thin-film electrodes, J. Phys. Chem. B. 34 (1999) 7184–7189. https://doi.org/10.1021/jp990066k
[66] Y. Hu, A. Kleiman-shwarsctein, A.J. Forman, D. Hazen, J. Park, E.W. Mcfarland, S. Barbara, S. Barbara, Pt-Doped α-Fe2O3 thin films active for photoelectrochemical water splitting, Chem. Mater. 20 (2008) 3803–3805. https://doi.org/10.1021/cm800144q
[67] L. Wang, W.A. Daoud, BiOI/TiO2-nanorod array heterojunction solar cell : Growth, charge transport kinetics and photoelectrochemical properties, Appl. Surf. Sci. 324 (2015) 532–537. https://doi.org/10.1016/j.apsusc.2014.10.110
[68] S. Ge, K. Zhao, L. Zhang, Microstructure-dependent photoelectrochemical and photocatalytic properties of BiOI, J. Nanoparticle Res. 14 (2012) 1015. https://doi.org/10.1007/s11051-012-1015-1
[69] H. Huang, K. Liu, Y. Zhang, K. Chen, Y. Zhang, N. Tian, Tunable 3D hierarchical graphene–BiOI nanoarchitectures: Their in situ preparation, and highly improved photocatalytic performance and photoelectrochemical properties under visible light irradiation, RSC Adv. 4 (2014) 49386–49394. https://doi.org/10.1039/C4RA07533A